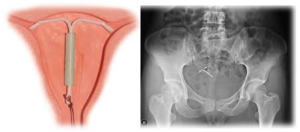
Amid calls to withdraw the Mirena IUD from market in the face of risks the birth control device could cause, Mirena lawsuit counts continue to grow. The IUD contraceptive once a favorite of women across all ages is now in the thick of controversy for complaints of migration and surgical injury associated with it.
Mirena Lawsuit Not Dismissed
A recent Mirena lawsuit filed in a Minnesota federal court highlights how a 26-year-old woman suffered uterus damage and other complications even after the IUD was removed. In another instance, a New Mexico court allowed a Mirena migration and perforation claim to proceed despite delayed filing considering the gravity of the case.
Judge William P. Lynch of New Mexico overruled Bayer’s objection highlighting delayed submission of notice to allow plaintiff Melissa Beauprez to proceed with her Mirena lawsuit. The court acknowledged “a certain sloppiness and inattention to detail” on the part of the Mirena attorney representing the plaintiff, but refused to dismiss the claim.
Melissa had a Mirena IUD implant in 2009. She went for removal after having persistent back pain and other symptoms for two years. According to her Mirena lawyer, X-ray and CT scans revealed that she was a victim of IUD migration and doctors performed an invasive surgery to remove the device embedded in her abdomen. The Mirena lawsuit seeks strict liability citing “defective product, failure to warn consumers, concealment of information, breach of implied warranty, and fraudulent practice.”
The litigation was first filed by Beauprez’s Mirena attorney in July 2014 without complying with the procedure of “serving a copy of the claim notice to IUD manufacturer Bayer.” The court ordered the plaintiff to provide the defendant a copy of the notice “no later than Dec. 17, 2014, Plaintiff must either effect service or provide the Court with a written explanation why service has not been effected.” But technical problems resulted in the notice reaching to Bayer five days later on December 22.
The defendant made the delay an issue and sought the dismissal of the Mirena lawsuit. However, the court ruled over the objection and accepted the contention of plaintiff’s Mirena lawyer that there was no deliberate delay in serving the notice and it was due miscommunication between him and another out-of-state attorney.
CDC Report Confirms Mirena Pregnancy Complication Risk
According to a recent report by the U.S. Centers for Disease Control, women getting pregnant while using Mirena IUD face the enhanced risk of abortion and other complications. This makes it hard a choice for women who accidentally become pregnant as the birth control device slips away and migrates. If they go for surgical removal of the displaced IUD, the injury and complication risks are high. If they do not, they face equally danger of suffering from abortion and other serious complications.
The report cites the potential risk of septic abortion, pre-term delivery, and serious infections, such as chorioamnionitis, in women becoming pregnant following Mirena migration. Prepared by the Reproductive Health Division, it warns that presence of the device may expose the fetus to synthetic hormones and 80 percent such pregnancies end up in abortions. There are many Mirena lawsuits filed claiming fetal death, miscarriages, and ectopic pregnancy.
However, a 2013 report in the American Journal of Obstetrics and Gynecology cited the risk of complications endangering both mother and child when a Mirena removal surgery is performed on a pregnant woman. There are also instances where strings could not be located and no removal surgery was performed.
A Mirena lawsuit filed by an Alabama couple a few months ago demonstrated how a pregnancy with Mirena IUD inside could cause havoc. The displacement of the T-shaped IUD led to the accidental pregnancy and the mother did not undergo the removal surgery for fear of fatal risk to both mother and the fetus and also the strings were not there. The newborn child had “preterm delivery, right-sided diaphragmatic hernia, pulmonary hypertension, degenerated right pulmonary artery, a large patent ductus-arteriosis (PDA), and persistent fetal oxygenation” attributed to exposure to artificial hormone released by the IUD. The baby died within 36 hours.
Contact Our Mirena Attorney Today
If you have or any of your relatives has experienced IUD injuries, including uterus perforation, contact our Mirena injury lawyer or call on 1-800-632-1404 to explore options to get financial compensation for physical suffering, trauma, economic losses, and non-economic damages.